Electron Microscope Division, Cell Biology Core Lab

Staffs:
Research Specialist:
Assistants:
- Hsu, Chia-Me
- Yen, Hsiao-Jung
Committee:
- Dr. Yu, Tien-Shin(chair)
- Dr. Chiou, Jian-Geng
- Dr. Jauh, Guang-Yuh
- Dr. Verslues, Paul E.
- Dr. Wang, Chung-Ju
Contact:
Wann-Neng Jane, Research Specialist
Tel: 02-27871012; Fax: 02-27827954
Email: wnjane@gate.sinica.edu.tw
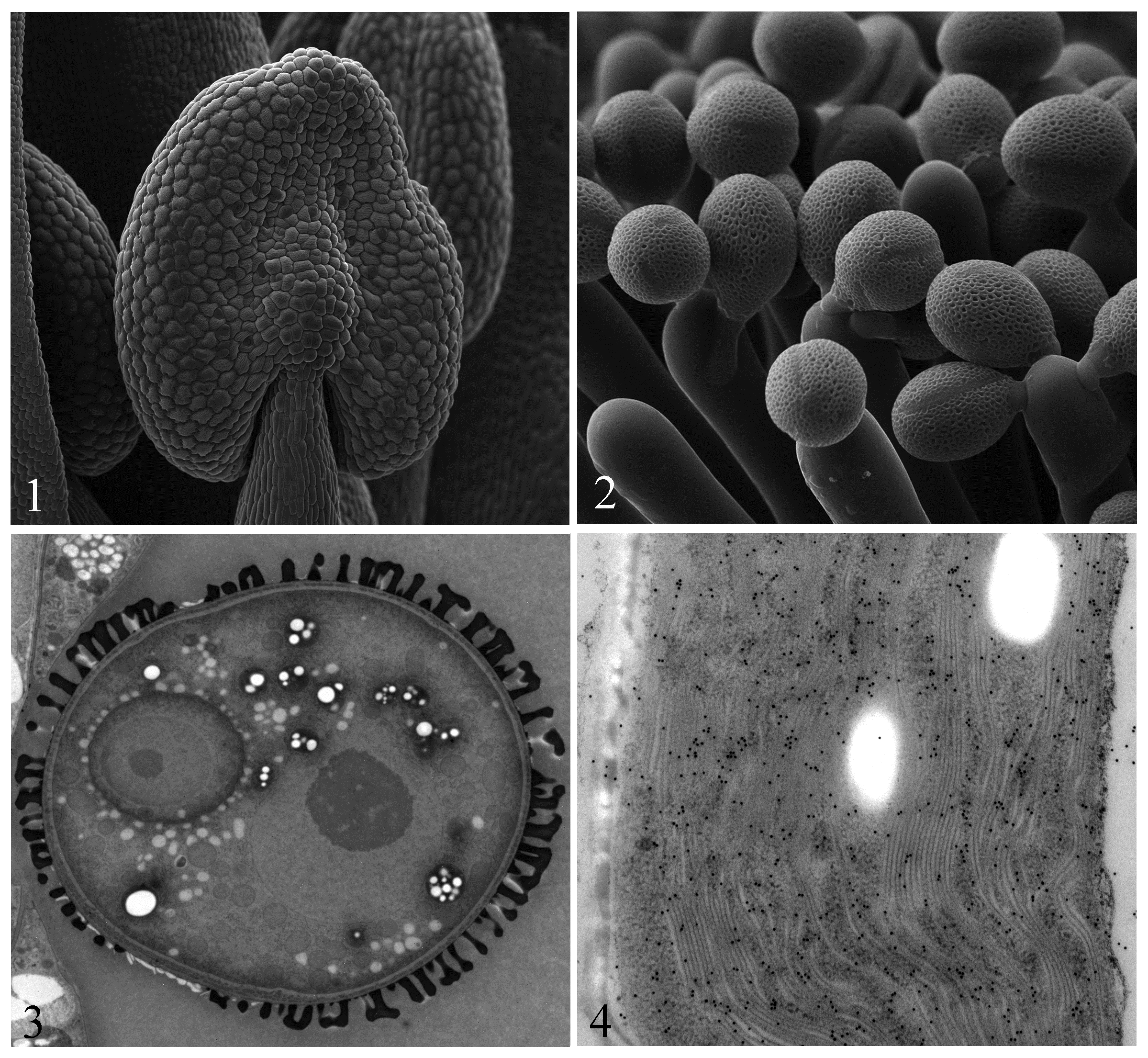
Fig.1 The young anther of Arabidopsis observed by Cryo-SEM.
Fig.2 The pollinating pollen grains of Arabidopsis observed by Cryo-SEM.
Fig.3 The bicellular pollen of Arabidopsis using high pressure freezing combined with freeze substitution.
Fig.4 The chloroplast of Nicotiana using high pressure freezing combined with freeze substitution and immunolabeling with anti-actin.
The Electron Microscope Division was established in 1972, and the lab had the second TEM in Taiwan. The lab’s mission is to provide up-to-date knowledge on EM methods for cell biology and immunocytochemistry, in particular the use of cryogenic techniques. The staffs of The Electron Microscope Division are comprised of one research specialist and two research assistants. Their work is including: maintaining the equipments and the laboratory for sample preparation, microtomy, and various cryogenic methods; and training new users to make best use of our equipments and various techniques; and assisting users in choosing the right methods and protocols for their research; and supplying a range of reagents specific for the relevant EM methods and protocols. The facilities are composed of a transmission EM, a cryo scanning EM, various equipments related to EM sample preparation, and two flow cytometers. We have various techniques related to electron microscopy and provide the related services, including ultramicrotomy, negative staining, shadow casting, freeze substitution, freeze etching, immunogold labeling, cryoSEM, high pressure freezing, and etc.
There are not chemical and mechanical damages in Cryo-SEM, and the sample preparation is quickly. It can observe the sample morphology like live and get the high quality of images (Fig. 1&2). The high pressure freezing combined with freeze substitution is the best method of TEM sample preparation. It can maintain sample orginal structure and keep off the chemical damages. It is the prefect selection for morphology (Fig. 3) and immunolabeling (Fig. 4).
Equipments available:
- Transmission Electron Microscope (FEI Tecnai G2 Spirit, 2014) with 4Kx4K CCD camea, STEM and tomography
- Scanning Electron Microscope: (FEI Quanta 200, 2007) with cryo system (Quorum PP2000TR FEI)
- High Pressure Freezer (Lecia EM PACT2, 2006)
- Freeze Fracture/Etching System (BAL-TEC BAF 060, 2008)
- Freeze Substitution System (Lecia EMAFS2, 2007, 2014)
- Ultramicrotomes: Lecia Reichert Ultracut S (1996); Lecia EM UC6 (2005, 2009); Lecia EM UR7 (2011)
- Freeze Dryer (BAL-TEC MED020, 2005)
- Critical Point Dryer (Leica EM CPD300, 2019)
- Ion Sputter (Hitachi E-1010, 2006)
- Carbon Coater (Cressington 208C, 2006)
Protocols of sample preparation:
- Chemical fixation, morphology
- Chemical fixation, immunolabeling
- Chemical fixation, low temperature imbedding, immunolabeling
- Cryo fixation, morphology
- Cryo fixation, immunolabeling
- Scanning electron microscopy

Transmission Electron Microscope
Cryo Scanning Electron Microscope

Freeze Fracture/Etching System

Ultramicrotome

High Pressure Freezer

Freeze Substitution System

Carbon Coater

Freeze Dryer

Critical Point Dryer

Ion Sputter