
Verslues, Paul E. (韋保羅)
Research Fellow
- Ph.D., Plant Biology, University of California, Riverside, USA
- Drought related signaling and metabolism
- paulv@gate.sinica.edu.tw
- paulv@as.edu.tw
- +886-2-2787-1077 (Lab: R426)
- +886-2-2787-1186 (Office: R426)
- Academia Sinica Archive
- ORCID
- Web of Science (WOS)
- Google Scholar
Drought stress is the most severe limitation to plant productivity worldwide and is of increasing concern because of climate change. Many physiological traits important for growth and maintenance of yield during drought remain surprisingly poorly understood at the cellular and biochemical level. The Verslues laboratory works to uncover the cellular signaling and biochemical mechanisms that underly plant drought resistance at the physiological level. More generally, our understanding of plant biology lags behind that of other organisms. For example, even in Arabidopsis thaliana, the most intensively studied model plant, one third of proteins are of unknown cellular function and up to 70% of those are plant-specific proteins (Reiser et al., 2024, Genetics, 227: iyae027). This lack of knowledge of how plant cells work is both of academic and practical interest as it restricts the use of tools such as gene editing to improve plant stress resistance and agronomic traits. Our genetic and biochemical analysis of drought (low water potential) resistance often leads us to previously unstudied or poorly annotated proteins. Thus, another focus of our research is to establish the cellular and biochemical roles of unknown function plant proteins. Examples include our recent discovery that NPH3-domain proteins are a new, plant-specific type of GTPase (Upadhyay-Tiwari et al. 2024) as well as the unexpected microtubule binding and stabilizing ability of MASP1 (Bhaskara et al., 2017). By combining drought physiology with cellular and biochemical methods, our research contributes to fundamental plant biology while also revealing cellular mechanisms potentially useful for plant improvement.
Ongoing research in the Verslues laboratory can be summarized in three main foci.
1. NPH3-domain proteins and NRL5: Understanding a new class of GTPase and using it to discover mechanisms of trafficking, cellular polarity, and drought sensing/signaling.
The Verslues laboratory established a forward genetic screen for drought/low yw-related mutants using a Proline Dehydrogenase1 promoter:luciferase reporter (ProDH1pro:LUC; Shinde et al., 2016). ProDH1 was selected because it responds to a combination of metabolic- and stress-related signals and because regulation of ProDH1 is key for the dual roles of proline metabolism as the source of protective proline accumulation or a promoter of ROS accumulation and cell death (Verslues et al., 2023). Thus, a forward genetic screen based on the ProDH1 promoter offers a new window into stress signaling and metabolic regulation not seen in previous screens.
One of the mutants isolated from our ProDH1pro:LUC screen has a single amino acid change (P335L) in the Non-Phototrophic Hypocotyl3 (NPH3) domain of NPH3-RPT2-Like5 (NRL5). nrl5 mutants have extreme low yw hypersensitivity due to mis-regulated proline metabolism (Fig. 1). We further discovered that NRL5, and other NPH3-domain proteins, are a plant-specific type of GTPase involved in intracellular trafficking (Upadhyay-Tiwari et al. 2024; Verslues laboratory, unpublished data). A functional NPH3 domain and interaction with trafficking proteins are required for the distinct polar localization of NRL5. Ongoing work in our laboratory indicates that NRL5 is involved in multiple aspects of protein trafficking and secretion. The extreme low water potential hypersensitivity of nrl5-1 is also an important resource to understand drought-related sensing and signaling mechanisms through approaches such as suppressor screening. These efforts will uncover new mechanisms of trafficking regulation and polarity of broad interest for cell biology as well as new aspects of drought sensing, signaling and metabolic regulation.
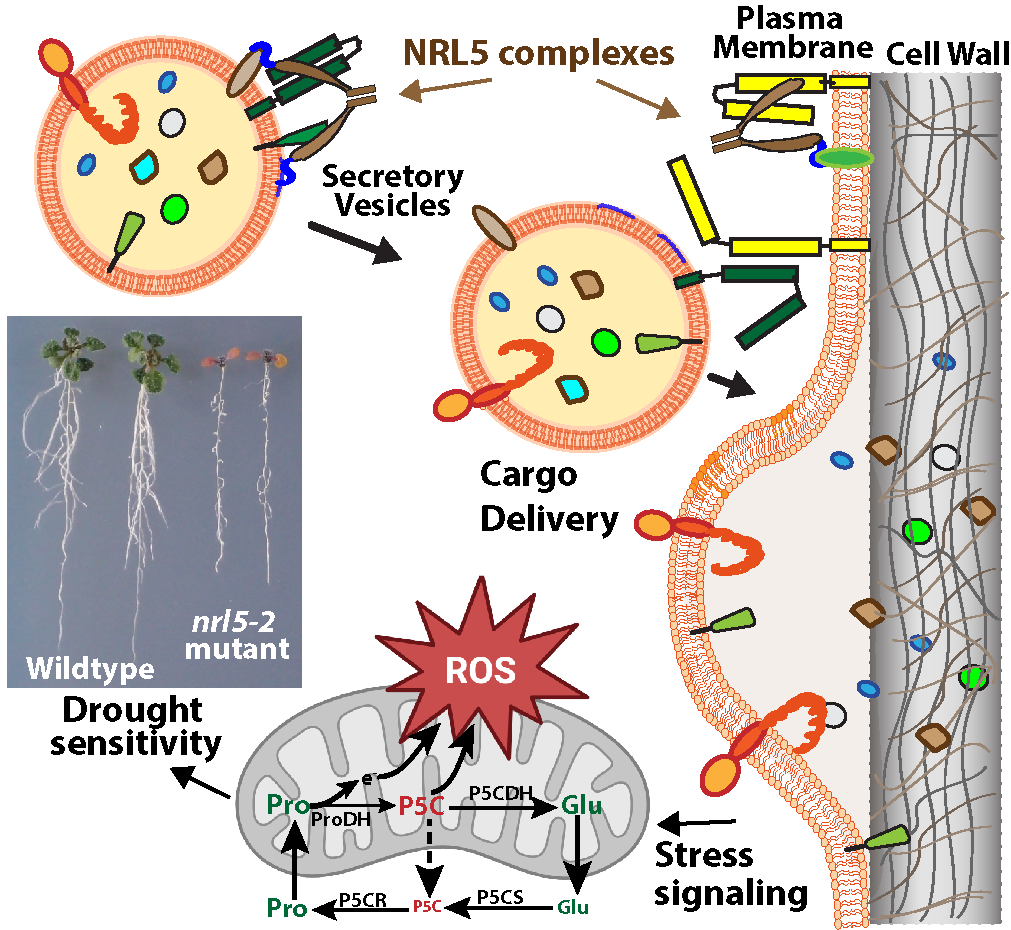
Fig 1: NRL5 trafficking function and the proline cycle. NRL5 has newly discovered GTPase activity and roles in intracellular trafficking. NRL5 mutants are dramatically hypersensitive to water limitation because altered trafficking to the cell wall and plasma membrane leads to mis-perception of the stress and mis-regulation of the proline cycle. Proline Dehydrogenase (ProDH) and P5C Synthetase (P5CS) are key stress-regulated components of the proline metabolism cycle that are mis-regulated in nrl5 and are components of our on-going research.
2. The EGR-MASP1 regulatory network controlling plant growth during drought stress.
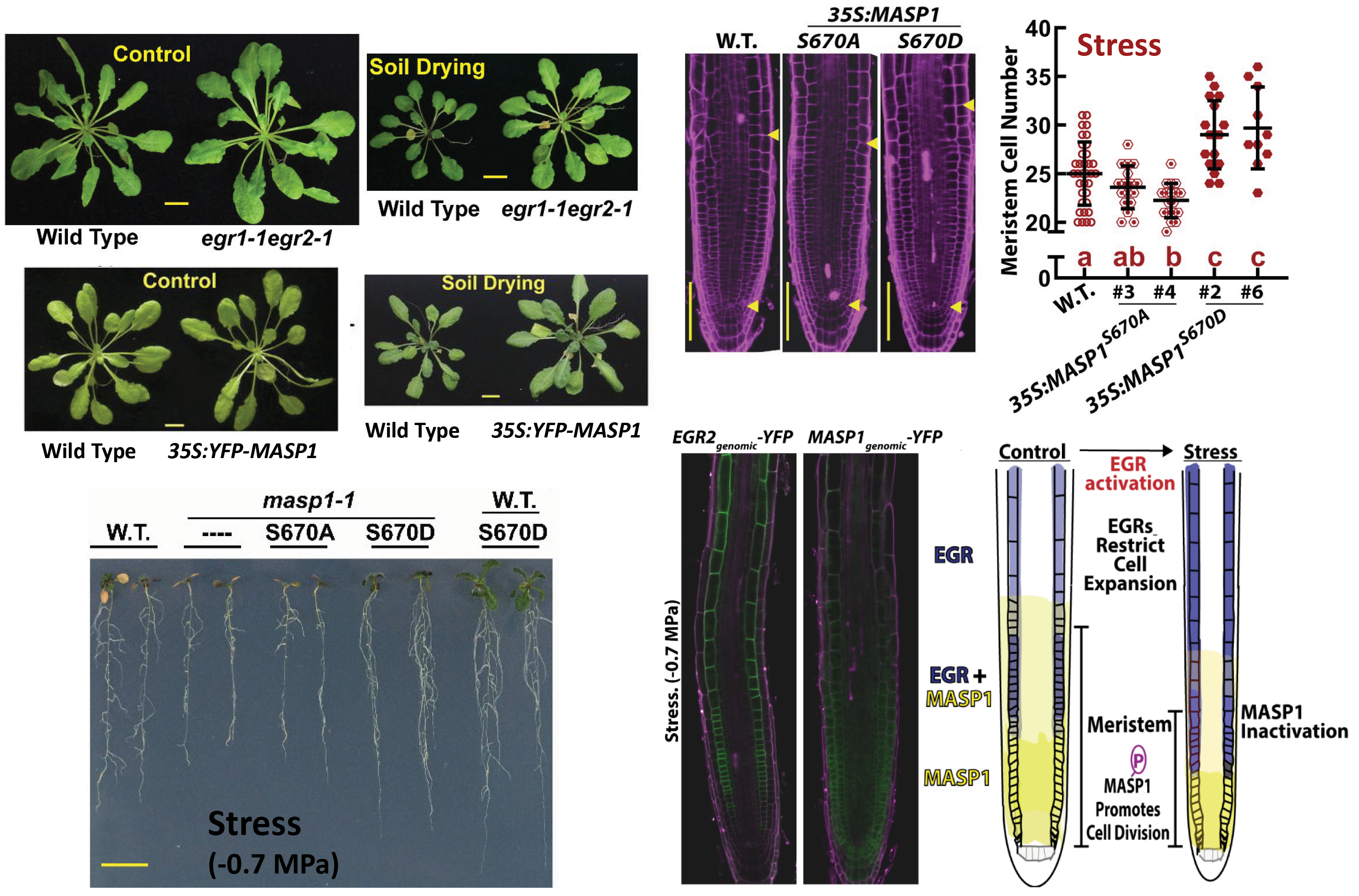
Fig. 2: EGR2 and MASP1 control plant growth by regulating meristem cell division and cell expansion. The EGR protein phosphatases are negative regulators that restrict growth (egr mutants grow more than wild type) while MASP1 is a negative regulator (ectopic MASP1 expression drives increased growth). MASP1 growth promotion function depends on its phosphorylation at a single site (S670) that is regulated by EGRs. Phosphomimic MASP1-S670D is hyperactive in promoting growth and preventing the reduction in root meristem size typically seen in drought stressed plants. Opposing gradients of MASP1 and EGR2 control meristem size and cell division activity during drought stress.
Three previously uncharacterized type 2C protein phosphatases (PP2Cs), which we named as the Clade E Growth-Regulating (EGR) PP2Cs, act as negative regulators to restrict plant growth during moderate severity drought/low yw stress. Phosphoproteomics of egr1-1egr2-1 identified Microtubule-Associated Stress Protein 1 (MASP1), that acts to promote growth during drought stress in a manner dependent upon EGR-regulated phosphorylation at a single critical site (S670). Phosphorylated MASP1 promotes growth by counteracting drought-induced reduction of meristem size (Fig. 2; Bhaskara et al., 2017; Longkumer et al., 2022).
EGR2 proximity labeling combined with phospho-proteomic analysis of egr1-1egr2-1 identified an interesting set of putative EGR-regulated proteins (Verslues laboratory, unpublished data). These include multiple classes of proteins involved in plasma membrane organization, transport or signaling (Fig. 3). Further research is investigating these putative EGR-regulated proteins and their roles in regulating plant growth during drought stress.
Similar experiments found putative MASP1 interaction with microtubule-associated proteins, consistent with MASP1 effect on microtubule stability, as well as potential cell cycle regulators that may preferentially interact with phosphorylated (or dephosphorylated) MASP1. Future work will identify proteins responsible for the repression of meristem cell division during drought, thus revealing new ways to improve plant growth maintenance during moderate severity water limitation.
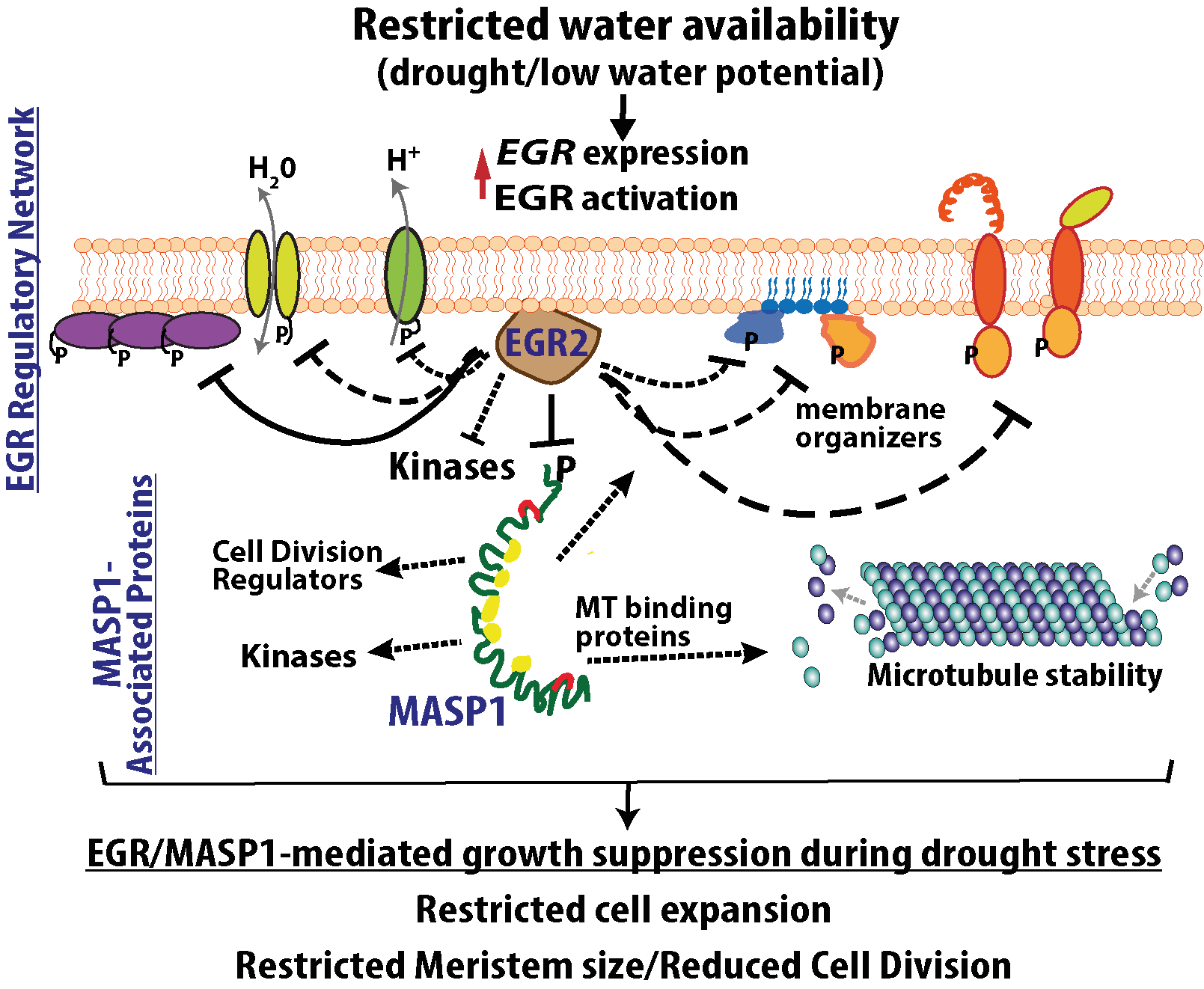
Fig. 3: The EGR2 and MASP1-associated proteins regulate plant growth, plasma membrane signaling, and microtubule dynamics.
3. The mysteries of proline metabolism: its regulation, connection to redox status, and contribution to drought resistance.
Proline metabolism is of interest in its own right as a contributor to plant stress resistance and also offers a window into stress signaling mechanisms. A focus on proline metabolism led us to identify EGRs and NRL5, thus we plan to continue investigating proline metabolism itself as well as using it to identify mechanisms of drought signaling and metabolic regulation. In addition to proline acting as a protective solute, the cycle of proline synthesis and catabolism (Fig. 1) is important for maintaining cellular redox status (Sharma et al., 2011; Verslues et al., 2023). In addition to ProDH1, the other key stress-regulated enzyme that controls flux through the proline cycle and is required for drought-induced proline accumulation is P5CS1. Little is known about P5CS1 regulation, in part because it has been difficult to express tagged P5CS1 and study its localization or interactions in transgenic plants. To circumvent this obstacle, the Verslues laboratory generated a P5CS1-YFP knock-in line (in-frame insertion of YFP at the endogenous P5CS1 locus) and demonstrated that P5CS1 is preferentially localized around chloroplasts (Longkumer et al., 2024). This resolved long standing questions about P5CS1 localization while also re-emphasizing old questions about the connection of proline metabolism to other metabolic pathways and to redox status of the chloroplast. As P5CS1 has been difficult to study in traditional transgenic plants, the P5CS1 knock-in also provides a new tool to investigate the factors that control P5CS1 expression and subcellular localization and identify P5CS1 interacting proteins. The Verslues laboratory also continues to investigate other regulators, particularly uncharacterized protein kinases and transporters, that influence drought-induced proline accumulation and drought resistance.
- Upadhyay-Tiwari N, Bau YC, Cao TTT , Longkumer T, Verslues PE* (2025) AFL1 is a phosphoinositide phosphate- and actin-binding protein. The Plant journal: for cell and molecular biology 124 (6), e70648
- Verslues PE* (2025) Understanding and Optimizing Plant Growth in Water-Limited Environments: “Growth versus Defense” or “Growth versus Risk mitigation”? Philosophical Transactions of the Royal Society B 380: 20240232.
- Verslues PE*, Upadhyay-Tiwari N (2024) Nonphototrophic hypocotyl 3 domain proteins: traffic directors, hitchhikers, or both? New Phytologist. https://doi.org/10.1111/nph.20211
- Upadhyay-Tiwari N, Huang XJ, Lee YC, Singh SK, Hsu CC, Huang SS, Verslues PE*(2024) The nonphototrophic hypocotyl 3 (NPH3) domain protein NRL5 is a trafficking-associated GTPase essential for drought resistance. Science Advances, 10(32):eado5429.
- Longkumer T, Grillet L, Chang HY, Luong TC, Chen CY, Putra H, Schmidt W, Verslues PE* (2024) Insertion of YFP at P5CS1 and AFL1 shows the potential, and potential complications, of gene tagging for functional analyses of stress-related proteins. Plant, Cell & Environment, 47 (6):2011-2026.
- Verslues PE* (2024) Please, carefully, pass the P5C. Journal of Experimental Botany, 75(3):663-666.
- Wong MM, Huang X-J, Bau Y-C, Verslues PE* (2024) AT Hook-Like 10 phosphorylation determines Ribosomal RNA Processing 6-Like 1 (RRP6L1) chromatin association and growth suppression during water stress. Plant Cell & Environment 47: 24-37
- Eckardt NA, Cutler S, Juenger TE, Marshall-Colon A, Udvardi M, Verslues PE (2023) Focus on climate change and plant abiotic stress biology. Plant Cell 35:1-3
- Juenger TE*, Verslues PE* (2023) Time for a drought experiment: Do you know your plants’ water status? Plant Cell 35:10-23
- Verslues PE*, Bailey-Serres J, Brodersen C, Buckley TN, Conti L, Christmann A, Dinneny JR, Grill E, Hayes S, Heckman RW, Hsu P-K, Juenger TE, Mas P, Munnik T, Nelissen H, Sack L, Schroeder JI, Testerink C, Tyerman SD, Umezawa T, Wigge PA (2023) Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant Cell 35:67-108
- Bhaskara GB, Lasky JR, Razzaque S, Zhang L, Haque T, Bonnette JE, Civelek GZ, Verslues PE, Juenger TE* (2022) Natural variation identifies new effectors of water use efficiency in Arabidopsis. Proceedings of the National Academy of Sciences USA 119: (33) e2205305119
- Verslues PE*, Longkumer T (2022) Size and activity of the root meristem: a key for drought resistance and a key model of drought-related signaling. Physiologia Plantarum 174: e13622
- Longkumer T, Chen C-Y, Biancucci M, Bhaskara GB, Verslues PE* (2022) Spatial differences in stoichiometry of EGR phosphatase and Microtubule-Associated Stress Protein 1 control root meristem activity during drought stress. Plant Cell 34: 742–758
- GL Chong, MH Foo, WD Lin, MM Wong, PE Verslues* (2019) Highly ABA-Induced 1 (HAI1)-Interacting protein HIN1 and drought acclimation-enhanced splicing efficiency at intron retention sites. Proceedings of the National Academy of Sciences 116(44):22376-22385.
- GB Bhaskara, MM Wong, PE Verslues* (2019) The flip side of phospho‐signalling: Regulation of protein dephosphorylation and the protein phosphatase 2Cs. Plant, cell & environment 42 (10), 2913-2930
- Kumar MN, Bau Y-C, Longkumer T, Verslues PE* (2019) Low water potential and At14a-Like1 (AFL1) effects on endocytosis and actin filament organization. Plant Physiology 179: 1594-1607
- Kalladan R, Lasky JR, Sharma S, Kumar MN, Juenger TE, Des Marais DL, Verslues PE* (2019) Natural variation in 9-cis-epoxycartenoid dioxygenase 3 and ABA accumulation. Plant Physiology 179: 1620-1631
- Wong MM, Bhaskara GB, Wen T-N, Lin W-D, Nguyen TT, Chong G-L, Verslues PE (2019) Phosphoproteomics of Arabidopsis Highly ABA-Induced1 identifies AT-Hook Like10 phosphorylation required for stress growth regulation. Proceedings of the National Academy of Sciences USA 116 (6): 2354-2363
- Kalladan R, Lasky JR, Chang TZ, Sharma S, Juenger TE, Verslues PE (2017) Natural variation identifies genes affecting drought-induced Abscisic Acid accumulation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA 114: 11536-11541
- Bhaskara GB, Nguyen TT, Yang T-H, Verslues PE (2017) Analysis of Phosphoproteome Remodeling After Short Term Water Stress and ABA Treatments versus Longer Term Water Stress Acclimation. Frontiers in Plant Science 8: 523
- Wong MM, Chong GL, Verslues PE (2017) Epigenetics and RNA processing: Connections to drought, salt and ABA? In: Methods in Molecular Biology, Plant Stress Tolerance: Methods and Protocols (Second Edition) vol 1631, Chapter 1. Sunkar, R ed, Springer
- Verslues PE (2017) Rapid quantitation of Abscisic Acid by GC-MS/MS for studies of abiotic stress response. In: Methods in Molecular Biology, Plant Stress Tolerance: Methods and Protocols (Second Edition), vol 1631, Chapter 21. Sunkar, R ed, Springer
- Bhaskara GB, Wen T-N, Nguyen TT, Verslues PE (2017) Protein Phosphatase 2Cs and Microtubule-Associated Stress Protein 1 control microtubule stability, plant growth, and drought response. Plant Cell 29: 169-191
- Des Marais D*, Juenger TE, Chang, TZ, Verslues PE, Lasky JR (2017) Interactive effects of water limitation and elevated temperature on the physiology, development, and fitness of diverse accessions of Brachypodium distachyon. New Phytologist 214(1): 132-144
- Verslues PE (2017) Time to grow: Factors that control plant growth during mild to moderate drought stress. Plant Cell & Environment 40: 177-179 (commentary article)
- Shinde S, Villamor JG, Lin W-D, Sharma S, Verslues PE (2016) Proline coordination with fatty acid synthesis and redox metabolism of chloroplast and mitochondria. Plant Physiology 172: 1074-1088.
- Verslues PE (2016) ABA and cytokinins: challenge and opportunity for plant stress research. Plant Molecular Biology 91:629-640 (review)
- Kumar MN1, Hsieh Y-F1, Verslues PE (2015) At14a-Like1 participates in membrane associated mechanisms promoting growth during drought in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA 112: 10545-10550 (1. Equally contributing authors)
- Bhaskara GB, Yang T-H, Verslues PE (2015) Dynamic proline metabolism: Importance and regulation in water limited environments. Frontiers in Plant Science6: 484. doi: 410.3389/fpls.2015.00484
- Lovell JT*, Mullen JL, Lowry DB, Awole K, Richards JH, Sen S, Verslues PE, Juenger TE, McKay JK (2015) Exploiting differential gene expression and epistasis to discover candidate genes for drought-associated QTLs in Arabidopsis thaliana. Plant Cell 27: 969-983
- Haswell ES, Verslues PE (2015) The ongoing search for the molecular basis of plant osmosensing. Journal of General Physiology 145: 389–394
- Kumar MN, Verslues PE (2015) Stress physiology functions of Arabidopsis Histidine Kinase (AHK) cytokinin receptors. Physiologia Plantarum 154: 369–380
- Wilson ME, Basu MR, Bhaskara GB, Verslues PE, Haswell ES (2014) Plastid hypoosmotic stress activates cellular osmotic stress responses. Plant Physiology 165: 119-128
- Verslues PE1, Lasky JR1, Juenger TE, Liu T-W, Kumar MN (2014) Genome wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis thaliana. Plant Physiology 164: 144-159 1. Co-first authors
- Sharma S, Shinde S, Verslues PE (2013) Functional characterization of an ornithine cyclodeaminase-like protein of Arabidopsis thaliana. BMC Plant Biology13:182
- Verslues PE, Bhaskara GB, Kesari R, Kumar MN (2013) Drought tolerance mechanisms and their molecular basis. In: Plant Abiotic Stress-Second Edition; Jenks, M.A. and Hasegawa, P.M. eds.; John Wilely and Sons, Inc. (review book chapter)
- Kumar MN, Jane W-N, Verslues PE (2013) Role of the putative osmosensor Arabidopsis Histidine Kinase 1 (AHK1) in dehydration avoidance and low water potential response. Plant Physiology 161: 942-953 (Reccomended by Faculty of 1000)
- Sharma S., Lin W, Villamor JG, Verslues PE (2013) Divergent low water potential response in Arabidopsis thaliana accessions Landsberg erecta and Shahdara. Plant Cell & Environment 36: 994-1008
- Bhaskara GB, Nguyen TT, Verslues PE (2012) Unique drought resistance functions of the Highly-ABA-Induced Clade A protein phosphatase 2Cs. Plant Physiology 160: 379-395
- Kesari R, Lasky JR, Villamor JG, Des Marais DL, Chen Y-JC, Liu T-W, Lin W, Juenger TE, Verslues PE (2012) Intron-mediated alternative splicing of Arabidopsis P5CS1 and its association with natural variation in proline and climate adaptation. Proceedings of the National Academy of Sciences USA 109: 9197-9202
- Sharma S, Villamor JG, Verslues PE (2011) Essential role of tissue specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiology 157: 292-304
- Verslues PE, Juenger TE (2011) Drought, metabolites and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Current Opinion in Plant Biology 14: 240–245
- Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. The Arabidopsis Book 8: 140
- Sharma S, Verslues PE (2010) Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell & Environment 33: 1838-1851
- Verslues PE (2010) Quantification of water stress-induced osmotic adjustment and proline accumulation for Arabidopsis thaliana molecular genetic studies. In R. Sunkar ed. Plant Stress Tolerance, Methods in Molecular Biology 639. Springer Science+Business Media, LLC. (methods book chapter)


Neha Upadhyay Tiwari 吳妮霞 Postdoctoral Fellow nehaut@gate.sinica.edu.tw

Hao-Yi Chang 張皓宜 Research Assistant haoyi1221@gate.sinica.edu.tw

I-Chen Chen 陳怡蓁 Research Assistant ichenchen@gate.sinica.edu.tw

Rishima Maheendran 芮熙瑪 Doctoral Student maheendran0001@gate.sinica.edu.tw

Thuy Thi-Thu Cao 高秋翠 Doctoral Student cao0001@gate.sinica.edu.tw

Dare Dolapo Oladele 歐拉德 Doctoral Student Oladele0001@gate.sinica.edu.tw

Shih-Shan Huang 黃詩珊 Lab manager manager426@gate.sinica.edu.tw
International
- 2022: 2022 Column Award for Distinguished Alumnus of the Division of Plant Science and Technology, College of Agriculture Food and Natural Resources, University of Missouri
Domestic
- 2020: Outstanding Research Award, Ministry of Science and Technology
- 2019: Investigator Award, Academia Sinica
- 2016: Outstanding Young Scholars Program , Ministry of Science and Technology
- 2014: Junior Research Investigators Award, Academia Sinica
- 2013: Ta-You Wu Memorial Award, National Science Council
- 2013: The Shang-Fa Yang Young Scientist Award, The Shang-Fa Yang Memorial Foundation
- 2013: Outstanding Young Scholars Program , National Science Council
- 2009: Career Development Award, Academia Sinica