[Erh-Min Lai] Glycine zipper-mediated effector translocation into target cells
POST:Type VI secretion systems (T6SSs) are contractile nanomachines used by bacteria to deliver effectors into target cells, enabling antagonism, virulence or nutrient acquisition. Antibacterial T6SS effectors exert their activity either in the periplasm, cytoplasm or on the membrane of target cells. While numerous antibacterial T6SS effectors have been identified, the underlying molecular mechanism by which T6SS and its associated effectors breach target cell membranes for translocation into cytoplasm still remains unknown. Understanding this mechanism provides implications over effector delivery and holds great importance for unravelling microbial interactions.
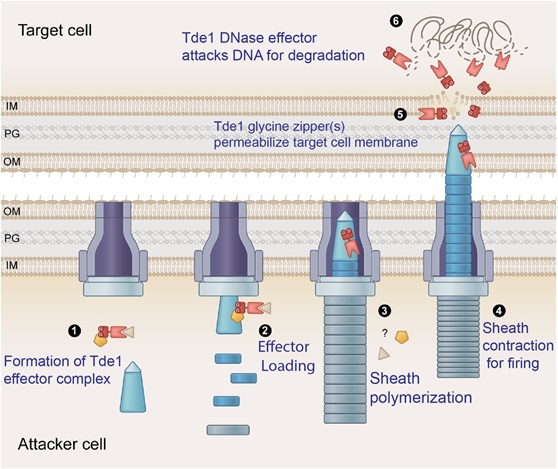
By studying the T6SS DNase effector 1 (Tde1) of a soil-borne phytopathogen Agrobacterium strain C58, the research team led by Dr. Erh-Min Lai uncovered that the effector itself plays an active role for entry into target prey cells for toxicity. The authors found that the N-terminus of Tde1 containing a single transmembrane domain overlapping with repeated glycine zipper motifs can cause membrane permeabilization in a G39xxxG43-dependent manner. Through co-immunoprecipitation, secretion, and DNase activity assays, the authors found that the N-terminus of Tde1 is necessary and sufficient for interaction with the adaptor protein Tap1 for secretion and the amino acid substitution of G39xxxG43 motif retains the Tde1 DNase activity and its interaction with Tap1 for secretion. Further, superfolder GFP fusion for translocation and interbacterial competition experiments showed that the G39xxxG43 motif of Tde1 is critical for target cell delivery and bacterial killing in DNase-dependent manner. Collectively, the authors discover that Tde1 DNase effector employs glycine zipper for translocation into target cells, representing a novel role of glycine zipper in effector delivery instead of toxicity.
Considering the widespread presence of glycine zipper motifs in bacterial effectors besides pore-forming toxins, the authors proposed that this glycine zipper-mediated delivery could be a common strategy deployed by many bacterial toxins for translocation across target cell membranes. This finding provides a theoretical basis of using the non-toxic effector module for the delivery of proteins of interests such as genetic modifiers into target cells. This strategy also offers an advantage over transforming foreign DNA for expressing a protein of interest from creating undesired genome manipulation.
This work is mainly contributed by the first author Dr. Jemal Ali, new Ph.D. graduate of the TIGP-MBAS program in National Chung-Hsing University and Academia Sinica. Other authors include Dr. Manda Yu, former Project Research Scientist and currently an Assistant Scientist at the University of Wisconsin-Milwaukee, Mr. Li-Kang Sung, Ph.D. student of the TIGP-MBAS program, Dr. Yee-Wai Cheung, Project Research Scientist and Dr. Erh-Min Lai, Research Fellow in IPMB. This work is published online in the EMBO reports.