[Guang-Yuh Jauh] HYCCIN2 is essential for recruiting the SH3P2-DRP1A complex to the cell plate in Arabidopsis embryos
POST: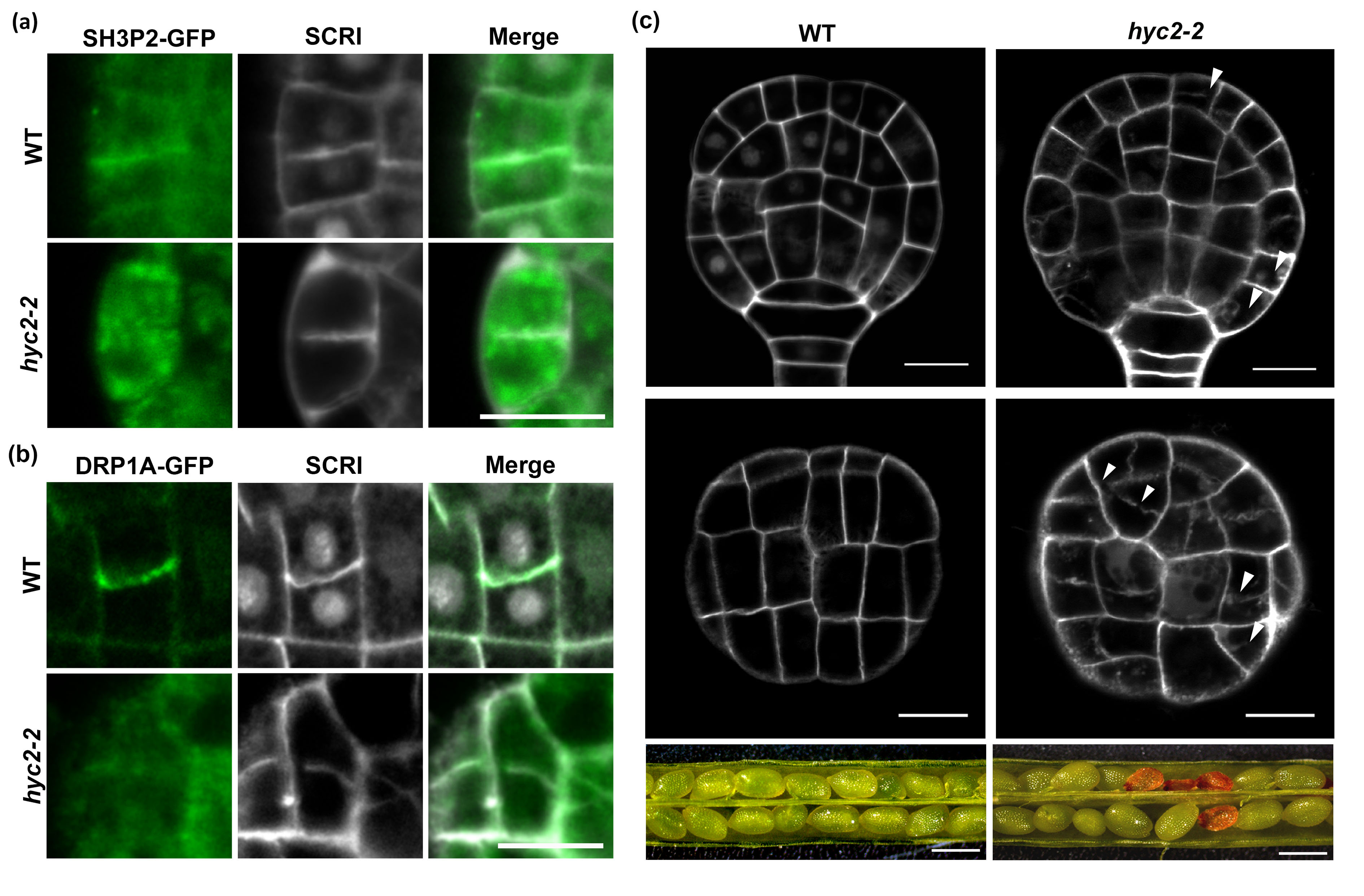
In Arabidopsis hyc2/- globular embryos, the localization of SH3P2 (a) and DRP1A (b) at the cell plate is compromised, leading to disrupted vesicle trafficking and membrane remodeling processes. As a consequence shown in (c), this impairment results in defective cell division patterns, abnormal embryo development, and ultimately contributes to a seed abortion phenotype, affecting plant reproductive success.
Cell division is a fundamental process essential for cellular proliferation. In plants, the formation of the cell plate—a structure unique to plant cytokinesis—differs markedly from mechanisms in animal cells due to the presence of a rigid cell wall. This process requires dynamic reorganization of the cytoskeleton and the targeted delivery of Golgi-derived vesicles, which collectively facilitate the construction of the new cell plate that separates the mother cell into two daughter cells. Although the structural aspects of plant cell division are well characterized, the molecular mechanisms that govern cytokinesis remain incompletely understood.
In a recent study by Hsu et al. from Dr. Guang-Yuh Jauh’s laboratory, HYCCIN2 (HYC2) was identified as a cytoskeletal crosslinking protein that localizes to the developing cell plate and contributes to both cell plate formation and phosphatidylinositol 4-phosphate (PI4P) production. Through genetic analysis and protein–protein interaction assays, the authors demonstrated that HYC2 associates with two known cytokinesis regulators, DRP1A and SH3P2. In hyc2/- mutant embryos, the localization of DRP1A and SH3P2 at the cell plate is impaired, accompanied by defective cell division during Arabidopsis embryogenesis. These findings reveal a critical role for HYC2 in orchestrating the recruitment of DRP1A and SH3P2 to the cell plate, thereby promoting proper cytokinesis and supporting normal embryo development in plants (Hsu et al., New Phytologist, 2025).