[Wan-Hsing Cheng] Interconnection between hexosamine biosynthesis and protein glycosylation: their roles and abiotic stress response in plants
POST: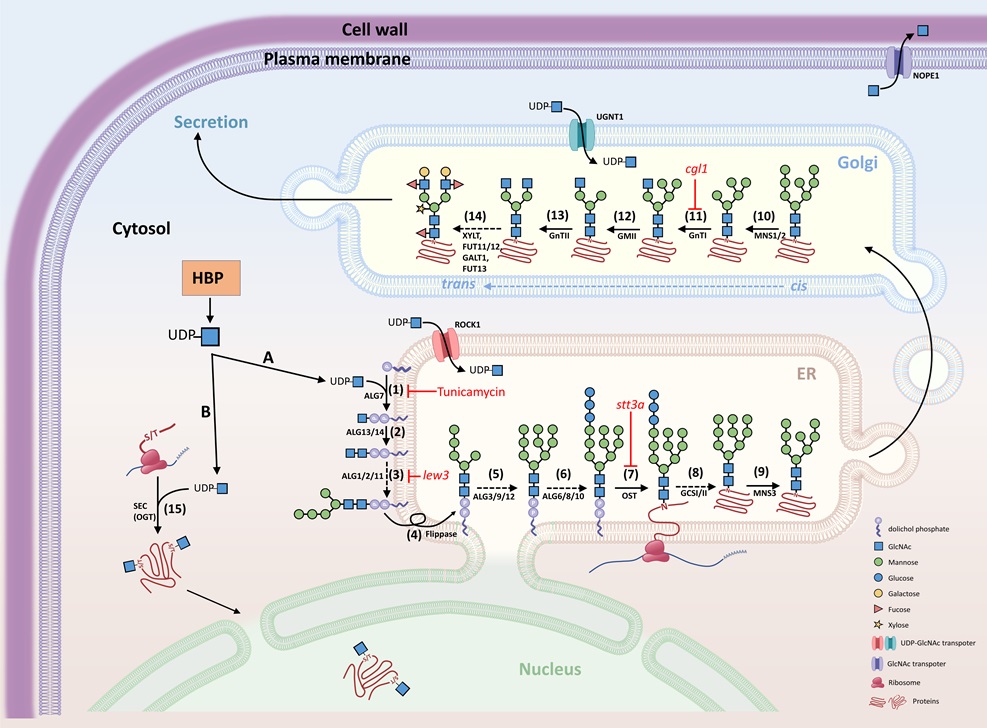
FIGURE. Interconnection between HBP and protein glycosylation (N-glycosylation and O-GlcNAcylation) A, N-glycosylation. UDP-GlcNAc is generated by the hexosamine biosynthesis pathway (HBP) and provides GlcNAc for the initial biosynthesis of oligosaccharide precursors at the cytosolic side of the ER. The oligosaccharide precursor (Man5GlcNAc2-PP-Dol) enters the ER lumen for N-glycan modification and N-glycosylation of proteins. Complex and hybrid N-glycan processing occurs in the Golgi apparatus. Proteins with mature N-glycans will be secreted to their destinations. B, O-GlcNAcylation. UDP-GlcNAc also provides the GlcNAc molecular unit directly to the Ser/Thr amino acids of proteins localized in the cytosol and nucleus.
N-acetylglucosamine (GlcNAc) is the fundamental amino sugar moiety essential for the glycosylation of protein, lipid, and cell wall components. UDP-GlcNAc, an active form of GlcNAc, is synthesized through the hexosamine biosynthesis pathway (HBP). A complete block of HBP normally causes lethality in any life form, reflecting the vital role of HBP in the normal growth and development of organisms. As HBP utilizes substrates including fructose-6-P, glutamine, acetyl-CoA, and UTP, it is capable of integrating endogenous nutrient/energy metabolites to better suit internal growth and development and external environmental stimuli. Thus, HBP is considered a sensing hub that may reprogram metabolic pathways to adapt to deleterious environmental challenges. In this review, we update the latest advances in the function of HBP and its connection with protein glycosylation (N-glycosylation and O-GlcNAcylation) and their response to abiotic stress. This paper was initiated and written by Drs. Ya-Huei Chen (Postdoc) and Wan-Hisng Cheng.