[Tuan-Hua David Ho] Chaetomella raphigera β-glucosidase D2-BGL has intriguing structural features and a high substrate affinity that renders it an efficient cellulase supplement for lignocellulosic biomass hydrolysis
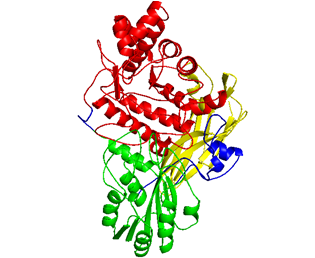
POST:To produce second-generation biofuels and other valuables chemical compounds, enzymatic catalysis is required to convert cellulose from lignocellulosic biomass into fermentable sugars. b-glucosidases finalize the process by hydrolyzing cellobiose into glucose, so the efficiency of cellulose hydrolysis is largely dependent on the quantity and quality of these enzymes used during saccharification. Dr. Tuan-Hua David Ho and his research team have discovered the fungal b-glucosidase D2-BGL from a Taiwanese indigenous fungus Chaetomella raphigera. (Kao et al. 2019 Biotechnology for Biofuels). Recombinant D2-BGL expressed in yeast displayed significantly higher substrate affinity than the commercial b-glucosidase Novozyme 188 (N188). Compared to N188, use of D2-BGL halved the time necessary to produce maximal levels of ethanol by a semi-simultaneous saccharification and fermentation process. When combined with Trichoderma reesei cellulases, it hydrolyzed acid-pretreated lignocellulosic biomasses more efficiently than the commercial cellulase mixture CTec3. Crystal structure analysis revealed that D2-BGL belongs to glycoside hydrolase (GH) family 3. The F256 substrate-binding residue in D2-BGL is located in a shorter loop surrounding the active site pocket relative to that of Aspergillus b-glucosidases, and this short loop is responsible for its high substrate affinity toward cellobiose.
Link: https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-019-1599-0