
Ho, Chin-Min (何金敏)
Associate Research Fellow
- Ph.D., Biochemistry and Molecular Biology, University of California, Davis, USA
- Inter-organelle communication and cell division
- chmho@gate.sinica.edu.tw
- chmho@as.edu.tw
- +886-2-2787-1069 (Lab: A423)
- +886-2-2787-1181 (Office: A431)
- Lab Website
- Academia Sinica Archive
- ORCID
- Web of Science (WOS)
- Google Scholar
Leaf epidermal development
As the interface between plants and the environment, the leaf epidermis provides the first layer of protection against dehydration (drought), UV and pathogen attacks through highly coordinated and functionally specialized cells in the epidermis, such as stomata, pavement cells and trichomes. Stomata, microscopic pores in the epidermis, function as valves to mediate the exchange of CO2 entering and water exiting the leaf through transpiration. Modulation of stomatal number could enhance drought tolerance of plants. More than half of Arabidopsis leaf epidermal cells are made through stomatal development. In stomatal development, asymmetric cell divisions produce meristemoids (red), precursors of stomatal guard cells, and stomatal lineage ground cells (SLGCs, blue) which may become pavement cells, or reinitiate asymmetric divisions to produce more stomata. How to maintain the multipotency in SLGCs? What drives a cell to exit from a proliferative state to a differentiation state? What is the developmental cue to specify the cell fates in leaf epidermis? The mechanism remains unclear.
By combining FACS-sorting, single cell sequencing, transcriptome analysis, molecular and cell biology approaches, we aim to identify factors in leaf epidermal development. Understanding the key players controlling this developmental plasticity could potentially generate the desired leaf epidermis for plants to resist the effects of climate change.
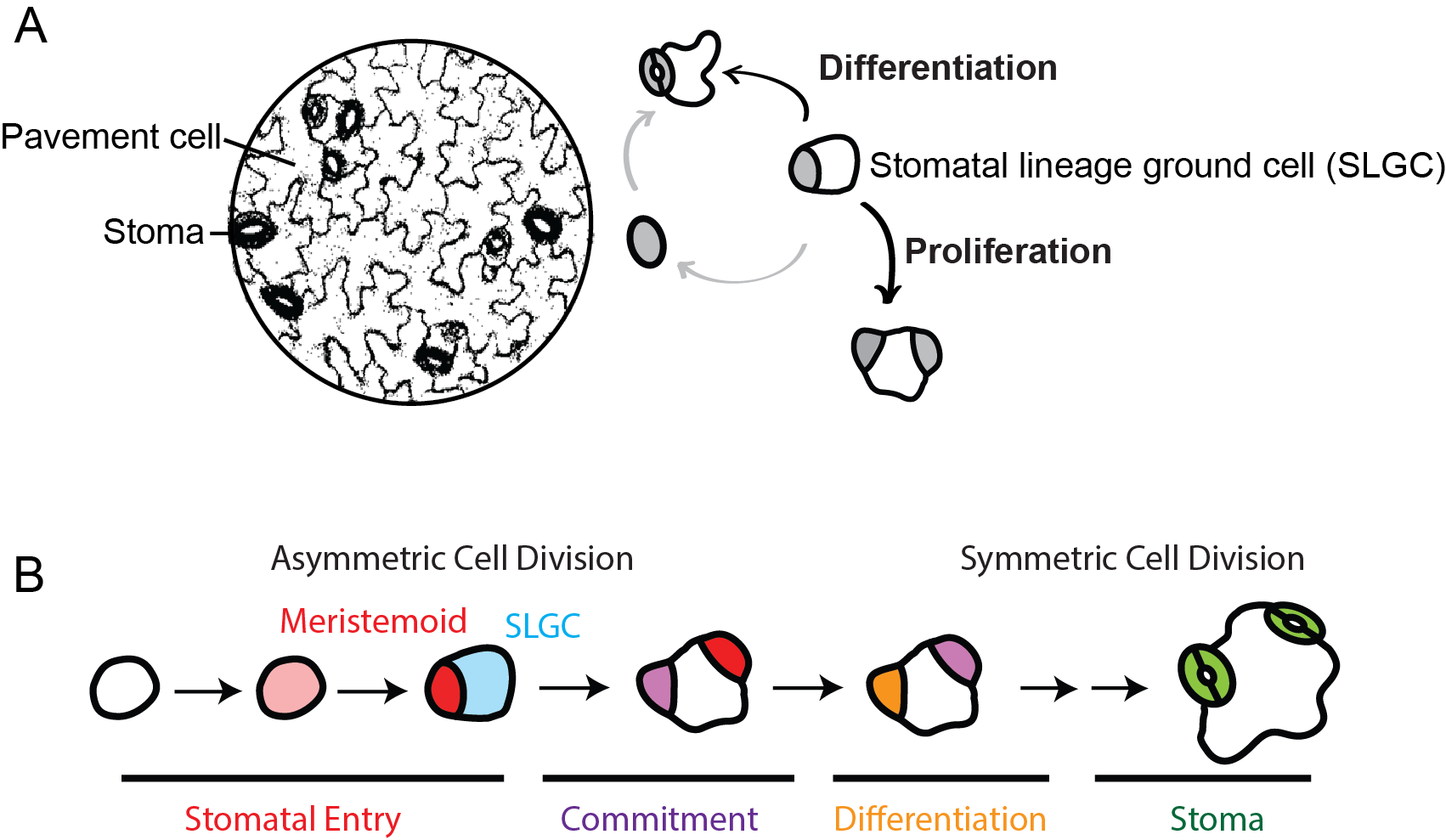
(A) Stomata and pavement cells are made from stomatal lineage ground cells (SLGCs) through proliferation or differentiation.
(B) A stomatal lineage is initiated from asymmetric cell division to produce two sister cells. The small cell (meristemoid, red) undergoes symmetric division to form a stoma. The large cell (SLGC, blue) could either undergo proliferation to produce more meristemoids, or differentiation to become a pavement cell.
Inter-organelle communication and its related signaling
Organelles have been treated as individual compartments with defined composition and organization in a cell. However, there is emerging evidence showing that inter-organelle communication through membrane contact sites is important for cellular functions and organismal homeostasis.
By using a forward genetic screen, we have found VST proteins play a role in ER-PM tethering. vst mutants exhibit stomatal phenotype and overall growth defects compared to wild type plants. The link between ER-PM (endoplasmic reticulum-plasma membrane) contacts and the receptor like kinase (RLK)-mediated developmental signaling prompts us to use the VSTs as an anchor to identify proteins that mediate the signaling in the tethering sites. The goal is to understand the function/organization of membrane contact sites and inter-organelle communication in the context of development and physiology in plants.
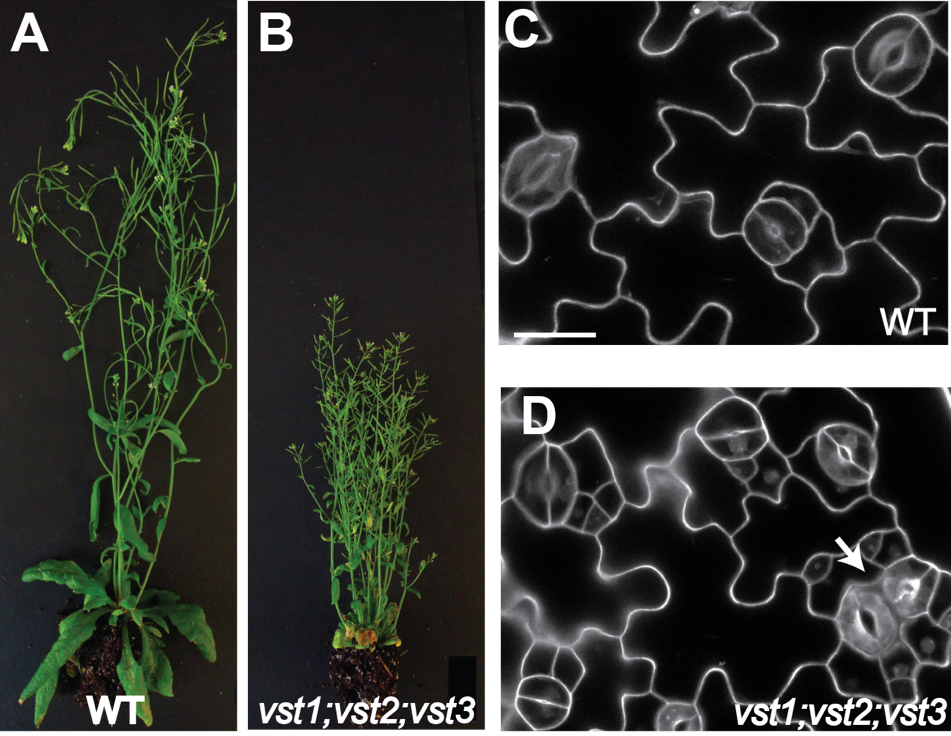
(A and B) vst triple mutant is shorter and more erect compared to WT. (C and D) There are excessive cell divisions and paired stomata in vst triple mutant (Ho et. al. 2016).
- Yang, S.-L., Tran, N., Tsai, M.-Y., and Ho, C.-M.K.* (2021) Misregulation of MYB16 causes stomatal cluster formation by disrupting polarity during asymmetric cell division. The Plant Cell --Focus Issue on Cell Biology. In press. (*Corresponding author). DOI: 10.1093/plcell/koab260
- Rovira-Clavé, X., Jiang, S., Bai, Y., Zhu, B., Barlow, G., Bhate, S., Coskun, A.F., Han, G., Ho, C.-M.K., Hitzman, C., et al. (2021). Subcellular localization of biomolecules and drug distribution by high-definition ion beam imaging. Nature Communications 12, 4628.
- Ho, C.-M.K.*, Bringmann, M., Oshima, Y., Mitsuda, N., Bergmann, D.C*. Transcriptional profiling reveals signatures of latent developmental potential in Arabidopsis stomatal lineage ground cells. Proceeding of the National Academy of Sciences.118(17) e2021682118 (* Corresponding author).
- Ho, C.-M.K., Paciorek, T., Abrash, E., and Bergmann, D.C. (2016). Modulators of stomatal lineage signal transduction alter membrane contact sites and reveal specialization among ERECTA Kinases. Dev. Cell 38, 345–357.
- Hotta, T., Kong, Z., Ho, C.M.K., Zeng, C.J.T., Horio, T., Fong, S., Vuong, T., Lee, Y.R.J., and Liu, B. (2012). Characterization of the Arabidopsis augmin complex uncovers its critical function in the assembly of the acentrosomal spindle and phragmoplast microtubule arrays. The Plant Cell 24:1494-1509
- Ho, C.M.K., Kiyama, L., Liu, B. (2012). The Arabidopsis MAP65-3 Protein Cross-links Anti-Parallel Microtubules toward Their Plus Ends in the Phragmoplast via Its Distinct C-termianl Microtubule-binding Domain. The Plant Cell. 24, 2071-2085.
- Ho, C.M.K., Hotta, T., Guo, F., Roberson, R.W., Lee, Y.R., and Liu, B. (2011). Interaction of anti-parallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis. The Plant Cell. 23, 2909-2923.
- Ho, C.M.*, Hotta, T.*, Kong, Z.*, Zeng, C.J.*, Sun, J., Lee, Y.R., and Liu, B. (2011). Augmin Plays a Critical Role in Organizing the Spindle and Phragmoplast Microtubule Arrays in Arabidopsis. The Plant Cell. 23, 2606-2618 (* Equal contribution).
International
- 2023: 2023 Women's Young Investigator Travel Award, American Society of Plant Biologists (ASPB)
- 2023: EMBO Global Investigator, European Molecular Biology Organization (EMBO)
Domestic
- 2023: Shang-Fa Yang Young Scientist Award, The Shang-Fa Yang Memorial Foundation
- 2022: Career Development Award, Academia Sinica
- 2021: Outstanding Young Scholar Award, Taiwan Society of Plant Biologists