
Chu, Hsiu-An (朱修安)
Associate Research Fellow
- Ph.D., Biochemistry, University of California, Riverside, USA
- photosynthesis, photosystem II and cyanobacteria
- chuha@gate.sinica.edu.tw
- chuha@as.edu.tw
- +886-2-2787-1047 (Lab: R302)
- +886-2-2787-1169 (Office: R301)
- Academia Sinica Archive
- ORCID
- Web of Science (WOS)
- Google Scholar
Molecular Mechanisms of Photosynthesis and Photoprotection in Cyanobacteria and Plants
Photosynthesis in cyanobacteria and plants utilizes solar energy to convert atmospheric carbon dioxide into sugars and oxidize water into molecular oxygen. This important biological process supports almost all life on Earth. Our major research interest is to study the molecular mechanisms of photosynthesis and photoprotection in cyanobacteria and plants. Our specific research goals are (1) to study the photoprotective mechanism of cytochrome b559 in Photosystem II (PSII); (2) to reveal the physiological function of the Qc-site in PSII and to explore the potential application of mutant cyanobacteria for bioenergy production; (3) to perform comparative and functional genomic analysis on a novel strain of Taiwan hot-spring cyanobacterium Thermosynechococcus CL-1 (TCL-1). Global warming, food shortages, and renewable energy are some of the most urgent and challenging issues of our time. Our research objectives aim to address these challenging issues.
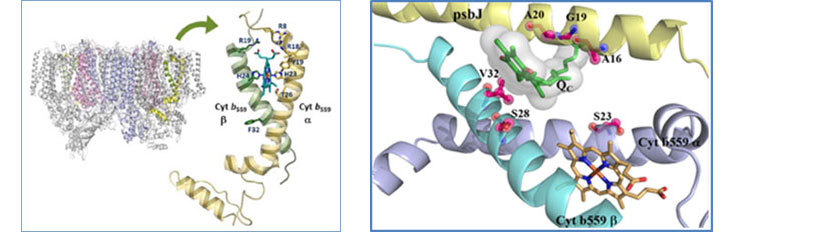
Tandem gene amplification restores photosystem II accumulation in cytochrome b559 mutants of cyanobacteria
We revealed a unique mechanism used by cyanobacteria to cope with mutation-induced instability of photosynthetic protein subunits. Cytochrome (Cyt) b559 is a key component of the photosystem II complex (PSII) that is essential for its proper functioning and assembly. Site-directed mutants of the model cyanobacterium Synechocystis sp. PCC6803 with mutated heme axial-ligands of Cyt b559 have little PSII and are thus unable to grow photoautotrophically. However, we identified two types of Synechocystis autotrophic transformants that retained the same mutations in b559 but are able to accumulate PSII and grow photoautotrophically. Through collaborations with Dr. Josef Komenda and coworker at the Czech Academy of Sciences, we demonstrated that the autotrophic transformants compensated for mutation-destabilized Cyt b559 subunits via multiple tandem amplifications of chromosomal segments containing the mutated psbEFLJ operon. Our results illustrate how tandem gene amplification restores PSII accumulation and photoautotrophic growth in Cyt b559 mutants of cyanobacteria, and may serve as an important adaptive mechanism for cyanobacterial survival. This work was recently published in New Phytologist (Chiu et al. 2022).
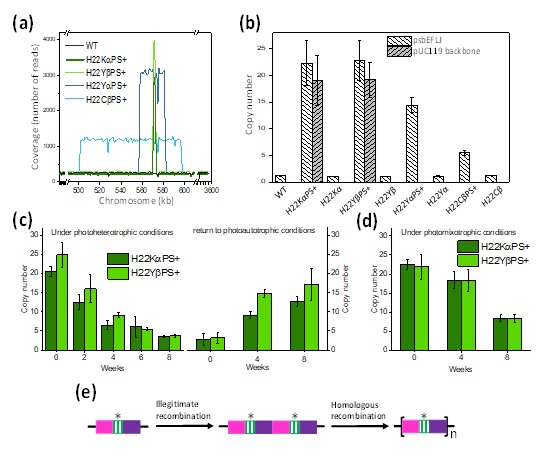
The Physiological Function of the QC Sitein Photosystem II and Application of Mutant Cyanobacteria for Bioenergy and Biochemical Productions
To reveal the physiological function of the QC site in PSII, we performed spectroscopic and functional characterization of several Cyt b559 and PsbJ mutant cells with mutations on and near the QC site in PSII in the model cyanobacterium Synechocystis PCC6803. Several mutant cells showed enhanced effects of state transitions under medium blue light and weakened effects of blue-light-induced NPQ under strong blue-light conditions. In addition, photosynthetic growth rate and biomass production were greater for several QC site mutant cells than WT under normal growth conditions. Our results suggest that the QC site of PSII may modulate the short-term light response in cyanobacteria. The improved photosynthesis and growth characteristics of mutant cyanobacteria may have a variety of applications such as bioenergy applications and productions of valuable commercial products (Huang et al., Biochemistry 2016 and Photosynthetica 2018; USA and Taiwan Patents).
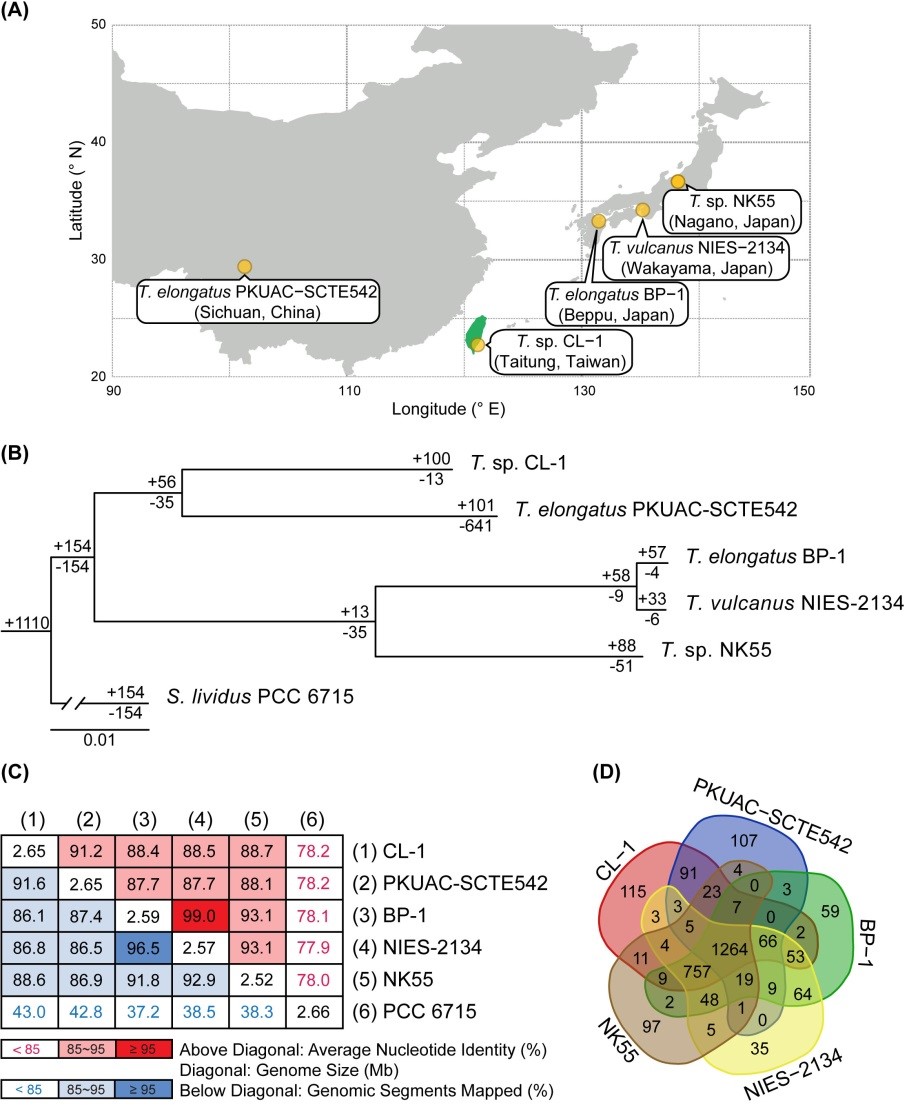
Comparative and Functional Genomic Analysis of a Novel Strain of Taiwan Hot-spring Cyanobacterium Thermosynechococcus sp. CL-1
Thermosynechococcus is a genus of thermophilic unicellular cyanobacteria that are dominant in microbial mats at about 50–65°C in alkaline hot springs of eastern Asia. A novel strain, Thermosynechococcus sp. CL-1, was collected from the Chin-Lun hot-spring (pH 9.3, 62°C) in eastern Taiwan and isolated by Dr. Hsin-Ta Hsueh at National Cheng-Kung University. Through the collaborations with Dr. Chih-Horng Kuo and coworkers in our institute, we complete the whole genome sequencing and comparative analysis across different species. Genome-scale phylogenetic analysis and average nucleotide identity (ANI) results suggested that CL-1 is a new species in the genus Thermosynechococcus. In addition, we identified distinct genetic differences between CL-1 and the other Thermosynechococcus strains. Our results suggest that CL-1 actively acquired many functional genes via horizontal transfer to cope with the various types of stresses in alkaline hot springs. The findings contributed to our understanding of the physiology, ecology, and evolution of thermophilic hot-spring cyanobacteria (Cheng et al., Frontiers in Microbiology 2020).
- Chen CN, Lin KM, Lin YC, Chang HY, Yong TC, Chiu YF, Kuo CH*, Chu HA* (2025) Comparative genomic analysis of a novel heat-tolerant and euryhaline strain of unicellular marine cyanobacterium Cyanobacterium sp. DS4 from a high-temperature lagoon. BMC Microbiology 25:279; https://doi.org/10.1186/
s12866-025-03993-7 - Chang HY, Yen HC, Chu HA, Kuo CH*. (2024) Population genomics of a thermophilic cyanobacterium revealed divergence at subspecies level and possible adaptation genes. Bot Stud 65, 35. https://doi.org/10.1186/s40529-024-00442-y
- Cheng YI, Lin YC, Leu JY, Kuo CH*, Chu HA* (2022). Comparative analysis reveals distinctive genomic features of Taiwan hot-spring cyanobacterium Thermosynechococcus sp. TA-1. Front Microbiol 13, 932840. https://doi.org/10.3389/fmicb.2022.932840
- Chiu Y-F and Chu H-A* (2022) New structural and mechanistic insights into functional roles of cytochrome b559 in photosystem II. Front. Plant Sci. 13:914922. (mini Review) doi: 10.3389/fpls.2022.914922
- Chiu YF, Fu HY, Skotnicová P, Lin KM, Komenda J*, Chu HA* (2022) Tandem gene amplification restores photosystem II accumulation in cytochrome b559 mutants of cyanobacteria, New Phytologist 233:766–780. doi: 10.1111/nph.17785
- Cheng YI, Chou Lin, Chiu YF, Hsueh HT, Kuo CH*, Chu HA*. (2020) Comparative genomic analysis of a novel strain of Taiwan hot-spring cyanobacterium Thermosynechococcus sp. CL-1. Front. Microbiol. 11:82.
- Endo K, Kobayashi K, Wang HT, Chu HA, Shen JR, Wada H* (2019) Site-directed mutagenesis of two amino acid residues in cytochrome b559 α subunit that interact with a phosphatidylglycerol molecule (PG772) induces quinone-dependent inhibition of photosystem II activity. Photosyn. Res. 139, 267–279, 2019-02.
- Huang JY, Hung NZ, Lin KM, Chiu YF, Chu HA* (2018) Regulating photoprotection improved photosynthetic growth and biomass production in the QC-site mutant cells of the cyanobacterium Synechocystis sp. PCC 6803. Photosynthetica 56, 192-199.
- Huang JY, Chiu YF, Ortega JM, Wang HT, Tseng TS, Ke SC, Roncel M, Chu HA* (2016) Mutations of cytochrome b559 and PsbJ on and near the QC site in photosystem II influence the regulation of short-term light response and photosynthetic growth of the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry 55:2214-2226.
- Chu HA*, Chiu YF (2016) The roles of cytochrome b559 in assembly and photoprotection of photosystem II revealed by site-directed mutagenesis studies. Front Plant Sci. 6:1261 (mini Review).
- Sheue CR*, Ho JF, Yao AW, Wu YH, Liu JW, Sauren D, Tsai CC, Chu HA, Chesson P and Ku MSB (2015) A Variation on Chloroplast Development: the Bizonoplast and Photosynthetic Efficiency in the Deep Shade Plant Selaginella erythropus. American J. Botany 102:1-12.
- Chu HA* (2013) Fourier transform infrared difference spectroscopy for studying the molecular mechanism of photosynthetic water oxidation. Front Plant Sci. 4:146 (mini Review).
- Chiu YF, Chen YH, Roncel M, Dilbeck PL, Huang JY, Ke SC, Ortega JM, Burnap RL and Chu HA* (2013) Spectroscopic and functional characterization of cyanobacterium Synechocystis PCC 6803 mutants on the cytoplasmic-side of cytochrome b559 in photosystem II. Biochim. Biophys. Acta Bioenergetics 1827:507-519.
- Chen YT, Shen CH, Lin WD, Chu HA, Huang BL, Kuo CI, Yeh KW*, Huang LC* and Chang IF* (2013) Small RNAs of Sequoia sempervirens during rejuvenation and phase change. Plant Biology. 15:27-36
- Chu HA, Chang IF, Shen CH, Chen YT, Wang HT, Huang LC* and Yeh KW* (2012) Photosynthetic properties and photosystem stoichiometry of in vitro-grown juvenile, adult, and rejuvenated Sequoia sempervirens (D. Don) Endl. Botanical Studies. 53:223-227.
- Wang TH, Chen YH, Huang JY, Liu KC, Ke SC* and Chu HA* (2011) Enzyme kinetics, inhibitors, mutagenesis and EPR analysis of the dual-affinity nitrate reductase in unicellular N2-fixing cyanobacterium Cyanothece sp. PCC8801. Plant Physio. Biochem. 49:1369-1376.
- Hou LH, Wu CM and Chu HA* (2011) Effects of ammonia on active water molecules of the oxygen-evolving complex in photosystem II as revealed by light-induced FTIR difference spectroscopy. Biochemistry 50:9248-9254.
