[Lay-Sun Ma] Maize Antifungal Protein AFP1 Elevates Fungal Chitin Levels by Targeting Chitin Deacetylases and Other Glycoproteins
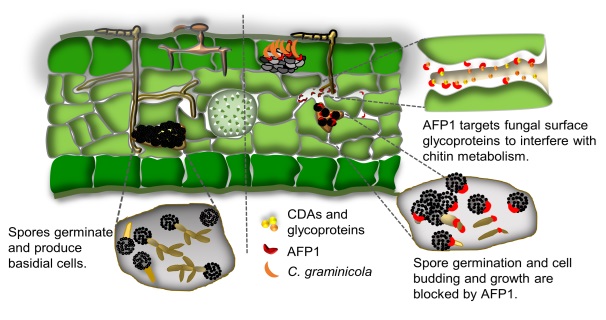
POST:Pathogenic fungi convert chitin to chitosan to evade plant perception and disarm chitin-triggered immune responses. Whether plants have evolved factors to counteract this evasion mechanism remains obscure. Here, we decipher the mechanism underlying the antifungal activity of maize secretory mannose-binding cysteine-rich receptor-like secreted protein (CRRSP), antifungal protein 1 (AFP1). AFP1 binds to multiple sites on the surface of sporidial cells, filaments, and germinated spores of the biotrophic fungus Ustilago maydis. It inhibits cell growth and budding, as well as spore germination. AFP1 promiscuously interacts with most chitin deacetylases (CDAs) by recognizing the conserved NodB domain to interfere with the enzyme activity. Deletion of O-mannosyltransferase 4 decreases protein mannosylation, which correlates with reduced AFP1 binding and antifungal activity, suggesting that AFP1 interacts with mannosylated proteins to exhibit an inhibitory effect. AFP1 also has extended inhibitory activity against Saccharomyces cerevisiae; however, AFP1 did not reduce binding to the double ΔΔcda1,2 mutant, suggesting the targets of AFP1 have expanded to other cell surface glycoproteins, probably facilitated by its mannose-binding property. Increasing chitin levels by modulating the activity of cell surface glycoproteins is a universal feature of AFP1 interacting with a broad spectrum of fungi to inhibit their growth.
Lay-Sun Ma, Wei-Lun Tsai, Florensia Ariani Damei, Raviraj M. Kalunke, Meng-Yun Xu, Yu-Han Lin, Hui-Chun Lee
Link: https://journals.asm.org/doi/epub/10.1128/mbio.00093-23