[Tuan-Hua David Ho] Improvements of the productivity and saccharification efficiency of the cellulolytic β-glucosidase D2-BGL in Pichia pastoris via directed evolution
POST: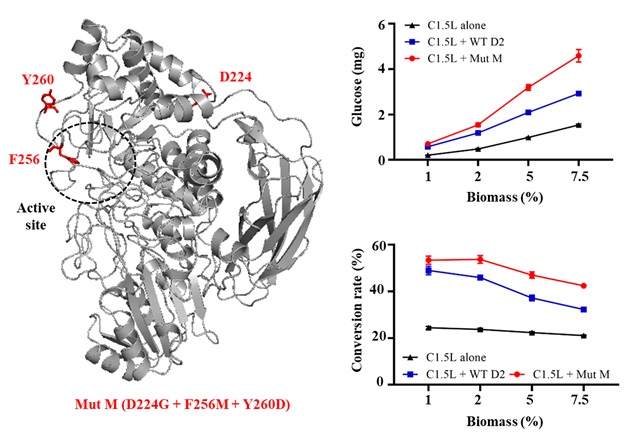
β-Glucosidase D2-BGL mutant Mut M (left) exhibits higher saccharification efficiency than wild-type D2-BGL (WT D2) at high sugarcane bagasse concentrations (right).
β-Glucosidases are essential for cellulose hydrolysis by catalyzing the final cellulolytic degradation of cello-oligomers and cellobiose to glucose. Dr. Tuan-Hua David Ho and his research team have discovered that the β-glucosidase D2-BGL, isolated from the fungus Chaetomella raphigera, has high substrate affinity and is an efficient β-glucosidase supplement to Trichoderma reesei cellulase mixtures for the saccharification of lignocellulosic biomass (Kao et al. 2019 Biotechnology for Biofuels). In this study, several D2-BGL mutants were generated following a directed evolution approach. The best mutant, Mut M, was constructed by combining three beneficial mutations. Expression of Mut M in Pichia pastoris resulted in higher production of recombinant protein, higher Vmax and greater substrate inhibition tolerance towards cellobiose relative to wild-type enzyme. Surprisingly, Mut M overexpression induced the endoplasmic reticulum (ER) unfolded protein response to a level lower than that with wild-type D2-BGL overexpression in P. pastoris, suggesting that Mut M could facilitate protein folding and sorting in ER. When combined with the T. reesei cellulase preparation Celluclast 1.5L, Mut M hydrolyzed acid-pretreated sugarcane bagasse more efficiently than wild-type D2-BGL.
https://biotechnologyforbiofuels.biomedcentral.com/articles/10.1186/s13068-021-01973-3